For decades, lung cancer has been the leading cause of cancer death in the world. Despite the possibilities of surgery and chemo- or radio-therapy treatments, only 15% of patients are still alive two years after diagnosis. This therefore represents both a medical challenge and a socio-economic problem at the forefront of public health concerns in Europe and the United States. ElyssaMed® will primarily target the cancer drug market. This market reached 60 billion Euro in 2012, including 6 billion in France. Its growth, estimated at 10.8% per year, is twice as fast as that of the entire pharmaceutical market.
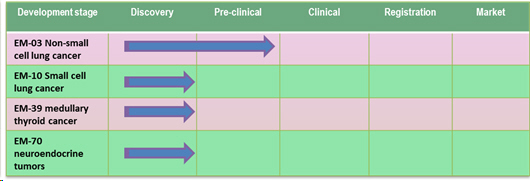
The first cancers that will be targeted by the ElyssaMed® technology are the non-small cell lung cancers which account for 80% of lung cancers. However, the team's research studies have shown that other cancers could be targeted by the vaccine including medullary thyroid cancers, neuroendocrine tumors and small cell lung cancers as well as all tumors that express CTPP.
The latter pathologies include those that are classified as rare (orphan) diseases for which a facilitated and accelerated development of new treatments is permitted.
Patents:
1) Patent technology
- Europe: issued under No. EP 2170941 28 December 2012
- China: issued under No. ZL 200880021020.X on 1 May 2013
- United States: issued under No. US 8,492,342 July 23, 2013
- Australia: issued under No. AU 2008277350 December 5 ,2013
- Japan: issued under No. JP 5393661 October 25, 2013
- Canada: application CA 2691406 patent pending
2) Patent Products
- European Patent Application 15176174.9 No. - 1403. Date: 09/23/15
ElyssaMed® develops multiple drug candidates targeting cancer based on unique technological platform; its lead product EM-03 is developed to treat non-small cell lung cancer.
- 1st in class
- Combination of selected proprietary immunogenic peptides targeting specific antigen of lung cancer.
- MOA : mimics the mechanism involve in the remission avoiding the TAP pathway
- Route of administration : Intra dermal injection
- Used in combination with marketed vaccine adjuvant.
- API & DP produced by CMO FDA / EMA approved